Nordic Bioscience receives Letter of Support from the FDA for the CPa9-HNE biomarker in inflammatory bowel diseases
Comunicato Precedente

Comunicato Successivo

"This trifecta of scientific excellence, high-quality biomarkers, and regulatory support sets us apart from the competition" commented Morten A. Karsdal, CEO of Nordic Bioscience. "The CPa9-HNE Letter of Support is not only another landmark achievement signifying our success story, but also something that our hard-working teams can be very proud of."
The CPa9-HNE biomarker measures a fragment of calprotectin S100a9, produced by human neutrophil elastase. It is detected in the serum and plasma of individuals with inflammatory bowel disease (IBD) and quantifies activated neutrophils, offering a non-invasive indicator of disease activity. In IBD trials, a frequent drug evaluation target is treating patients with moderate to severe ulcerative colitis or Crohn's disease. Neutrophils are important immune cells contributing to IBD, and CPa9-HNE can improve disease activity monitoring.
"The Letter of Supports underlines what we have sought to achieve with CPa9-HNE: this marker has the potential to be applied as a patient enriching tool for clinical trials in the future, by identifying patients more likely to have moderate or severe endoscopic disease activity" added Joachim Høg Mortensen, Scientific Director of Gastrointestinal Diseases, Nordic Bioscience. "This can significantly streamline patient recruitment, ensuring you target the right profiles. In addition, CPa9-HNE, as a serum calprotectin biomarker, provides a blood-based measure of disease activity, and has the potential to be used for monitoring of disease activity and treatment response."
Nordic Bioscience has a strong history in ECM remodeling research, earning FDA Letters of Support for its PRO-C3 and PRO-C6 biomarkers. The Company has transferred the FDA-approved bone biomarker CTX-I and fibrogenesis biomarker PRO-C3 to the Roche COBAS platform. With over 125 ELISA biomarkers developed to quantify unique ECM fragments, Nordic Bioscience's tools are used across all stages of drug development and patient selection in various diseases.
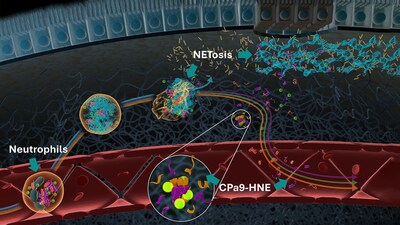
[1] CPa9-HNE is currently measured in hand-held ELISA assays, though development is ongoing for high precision automated platforms. Not for use in diagnostic procedures.
Contact: Elijah Aighobahi, [email protected], +45 4452 5252
![]() View original content:https://www.prnewswire.co.uk/news-releases/nordic-bioscience-receives-letter-of-support-from-the-fda-for-the-cpa9-hne-biomarker-in-inflammatory-bowel-diseases-302299933.html
View original content:https://www.prnewswire.co.uk/news-releases/nordic-bioscience-receives-letter-of-support-from-the-fda-for-the-cpa9-hne-biomarker-in-inflammatory-bowel-diseases-302299933.html





